Define System In Chemistry
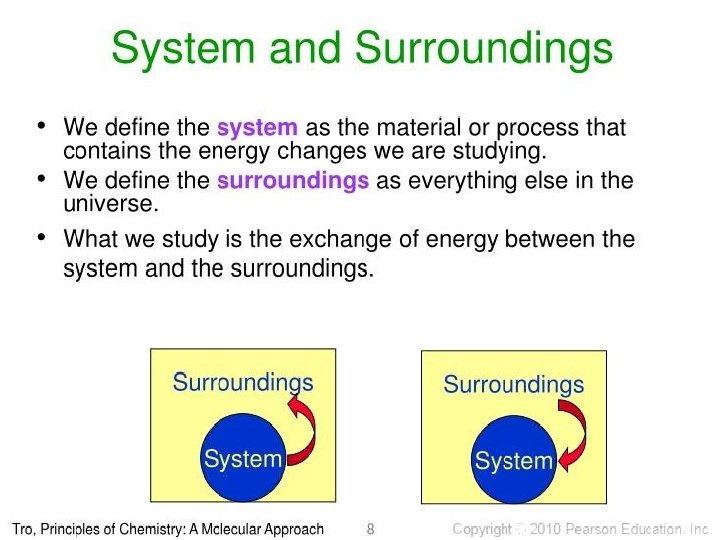
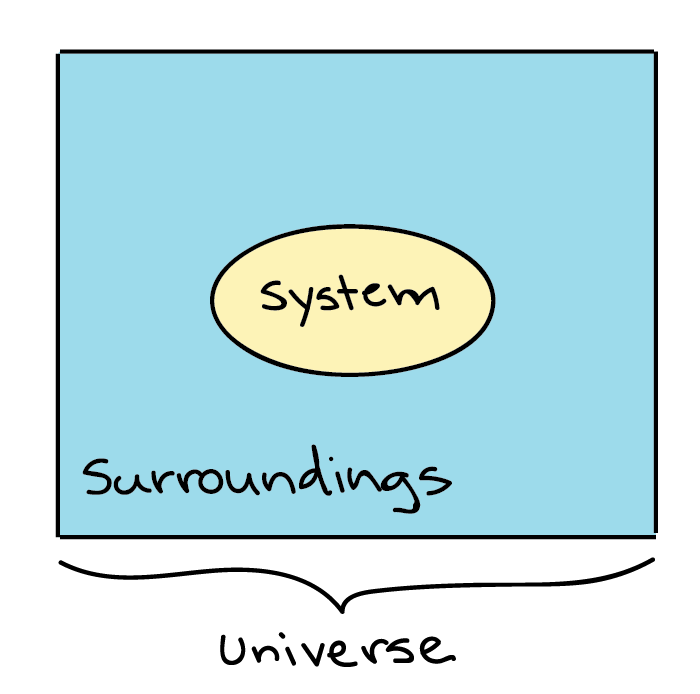
Define system in chemistry. To show a reaction or system is in equilibrium. The surrounding is everything else that is not the system defined. Thermodynamics science of the relationship between heat work temperature and energy.
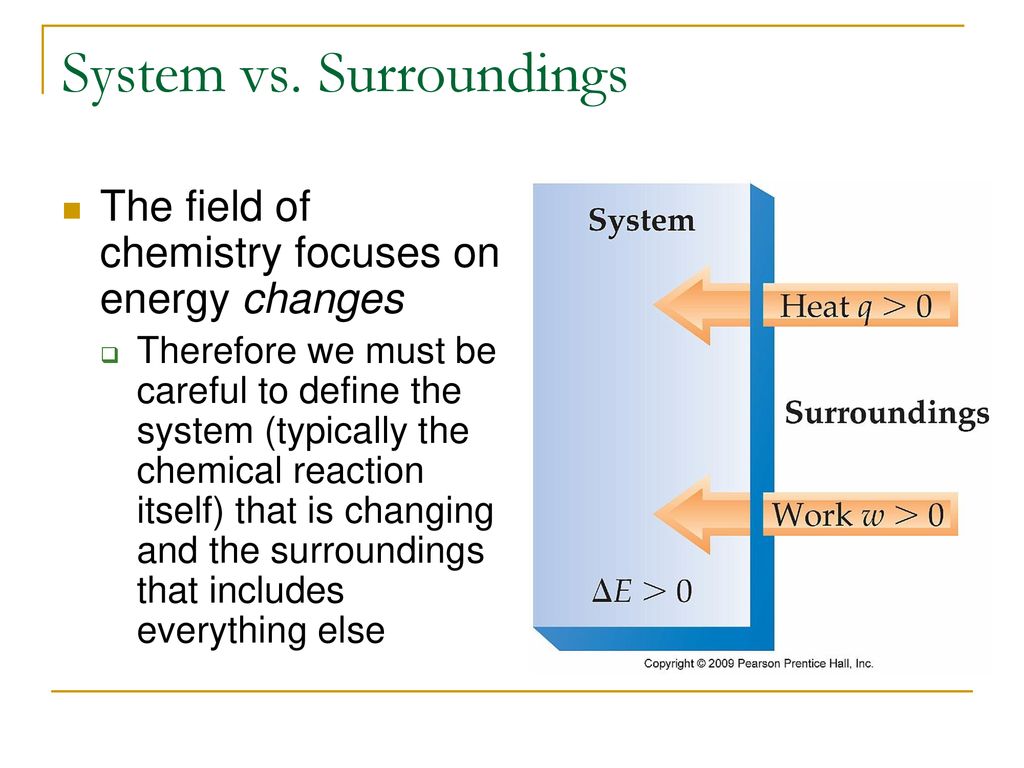
The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work. Definition of system - Chemistry Dictionary. 9 rows system - physical chemistry a sample of matter in which substances in different phases are.
What Is a System. What is a System. A system as it is defined in physics or chemistry is nothing more than a collection of objects or smaller systems that can be identified.
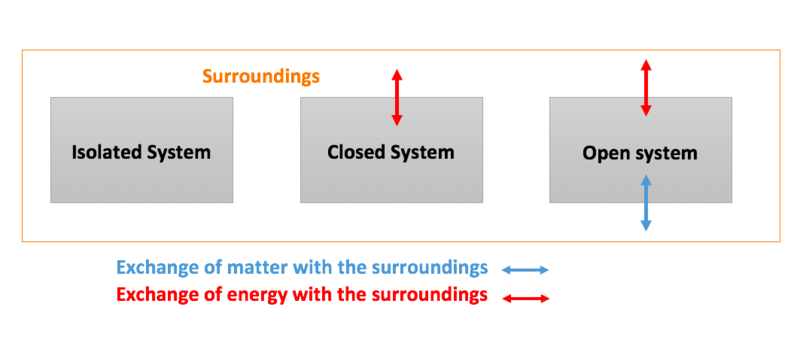
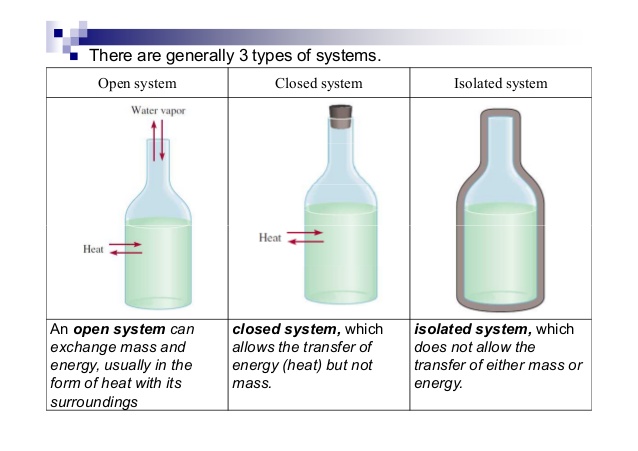
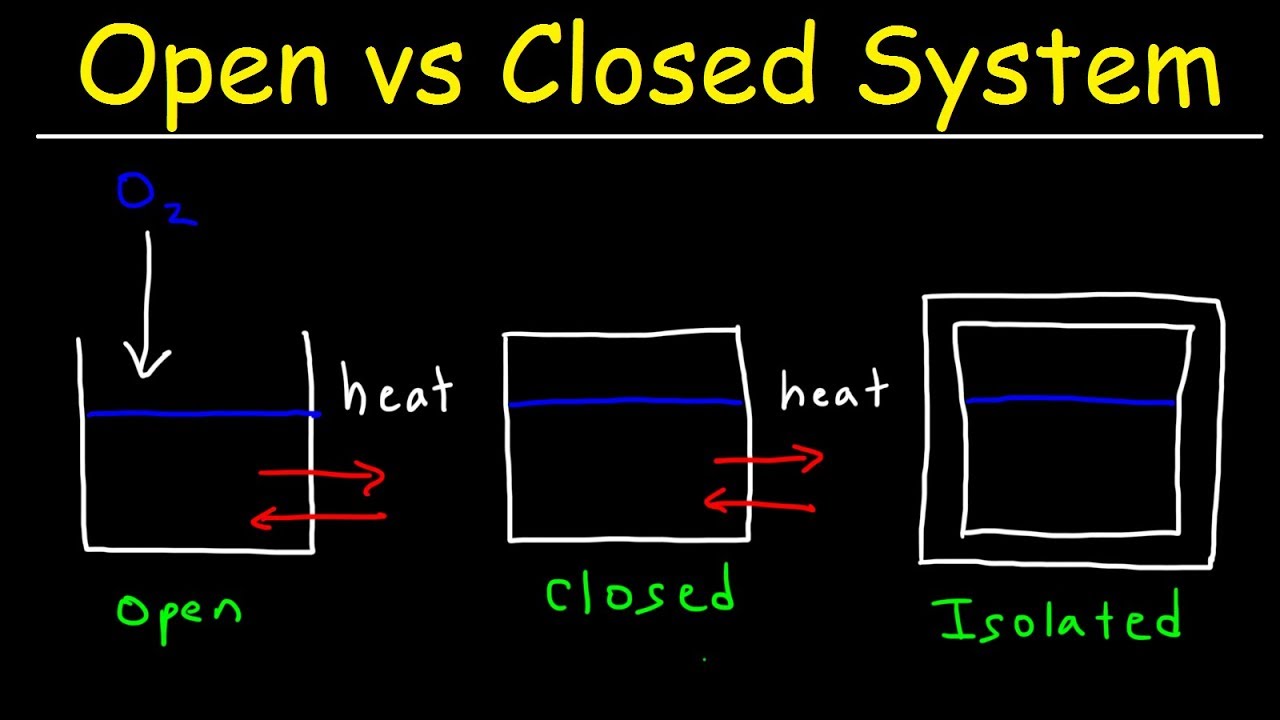
A system that does not allow the exchange of either energy or matter with the surroundings is called an isolated system. Once we have selected a set of independent variables consistent with the physical nature of the system and any conditions or constraints we can treat all other state functions as dependent variables whose values depend on the independent variables. Knowledge or a system of knowledge covering general truths or the operation of general laws especially as obtained and tested through the scientific method and concerned with the physical world and its.
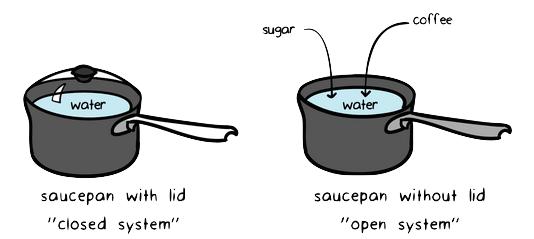
Degree of freedom tells about the input or no of parameters which are required to define a particular system. Degree of freedom is used to define a state of matter using different parameter. In chemistry a closed system is one in which neither reactants nor products can enter or escape yet which allows energy transfer heat and light.
Define system in chemistry On September 20 2021 Posted by In Uncategorized With No Comments Posted by In Uncategorized With No Comments. The system could be a car engine a mass of air in the atmosphere or even a soft drink can. Usually the word system refers to a collection that makes thinking about a problem more convenient.
Helmenstine Anne Marie PhD. This means that it can both absorb energy and have energy escape from its boundaries.
Classical thermodynamics looks at macroscopic aspects of matter.
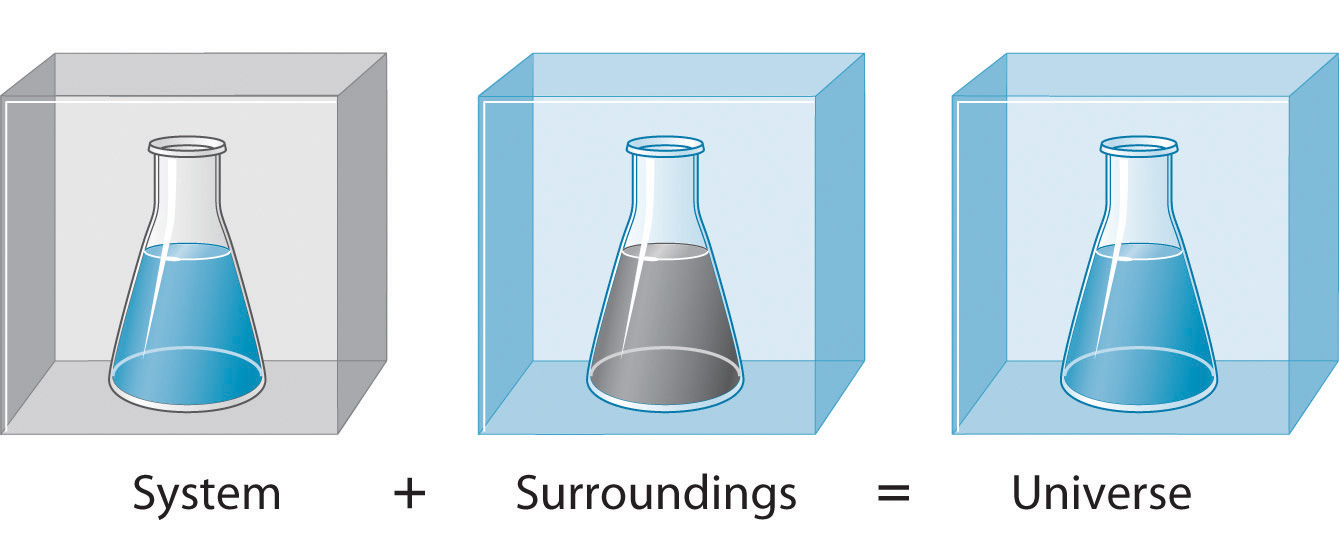
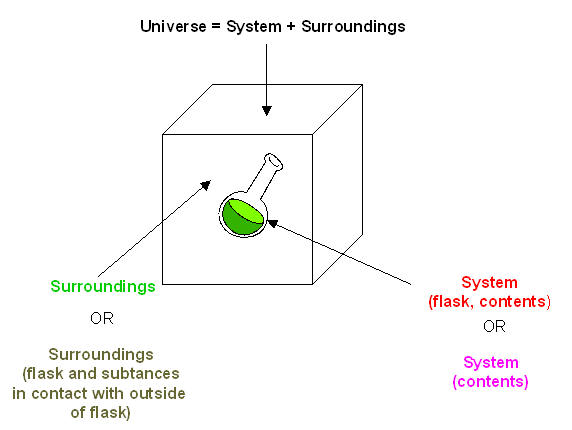
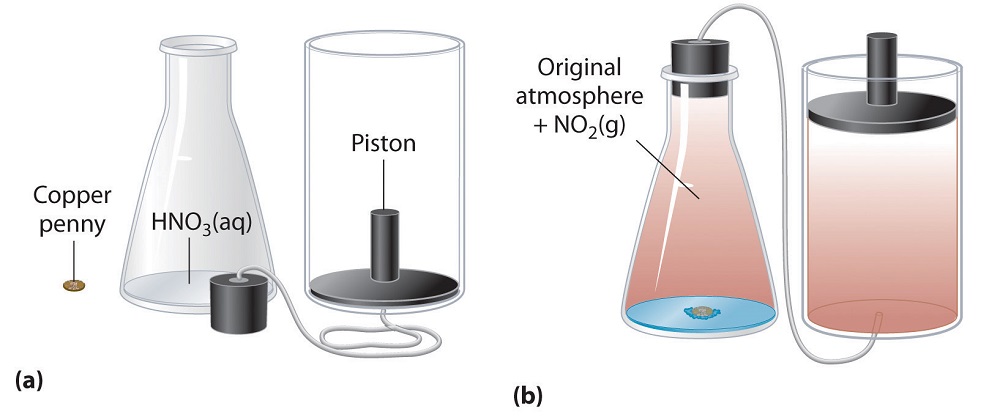
In the simple chemical equation below reactant A is in equilibrium with product B. Heat is lost to the surroundings to it may appear matter and energy are not conserved. Chemists are interested in systems containing matterthat which has mass and occupies physical space. The scientific discipline of chemistry includes a branch called thermodynamics that observes the changes that take place with different forms of energy. A system within thermodynamics is defined as part of the physical universe. In this branch the concepts of system and surroundings are used repeatedly. A system such as this which loses heat or other energy to its surroundings is also known as a dissipative system. In chemistry a closed system is one in which neither reactants nor products can enter or escape yet which allows energy transfer heat and light. Definition of system - Chemistry Dictionary.
In chemistry a closed system is one in which neither reactants nor products can enter or escape yet which allows energy transfer heat and light. The system has various inputs which go through certain processes to produce certain outputs which together accomplish the overall desired goal for the system. We can define the state of the system with the values of a certain minimum number of state functions which we treat as the independent variables. Definition of system - Chemistry Dictionary. It deals with the properties of aggregates of vast numbers of microscopic particles molecules atoms and ions. 9 rows system - physical chemistry a sample of matter in which substances in different phases are. A system such as this which loses heat or other energy to its surroundings is also known as a dissipative system.

















/the-5-branches-of-chemistry-603911-v1-25bffb5098484989a978951215bef01f.png)






/anhydrous-chemistry-definition-603387_final-46e5c09532c045568b0a07dfddba7397.png)
Post a Comment for "Define System In Chemistry"